All living cells require iron to live.
This includes bacteria and human cells.
Why is that?
Because iron has essential functions in the metabolism of every living cell.
The problem is, that free iron is actually toxic.
So, each organism binds iron to a special vehicle.
And bacteria were smart enough and developed strategies to steal those vehicles from other organisms.
Want to find out how and why?
Read on.
The iron in our blood
In the human body, we have many different iron-carrying vehicles. Most of the iron in our body is stored in a special molecule called haem. Haem has a ring structure with the iron (Fe) bound in the middle
It looks more or less like in this picture.

Haem is part of a protein called haemoglobin. And haemoglobin lives in the membranes of each of our red blood cells.
The iron inside the haem inside the haemoglobin is the one that binds oxygen and carries it to all our cells.
Interestingly, when haem is bound to oxygen, it turns red. And because the haem is red, our blood is red. Without the oxygen, the haem complex is blue-red.
Bacteria stealing iron
So, our body stores most of the iron within these haem molecules. And many pathogenic bacteria developed strategies to survive within the body by stealing our haem and thus our iron.
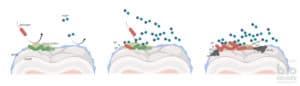
To do this, they produce special transporters that sit in the membrane of the bacterium. This transporter looks like a barrel, like the blue one in the figure below. This barrel tightly binds haem and transports it through the membrane into the bacterium.
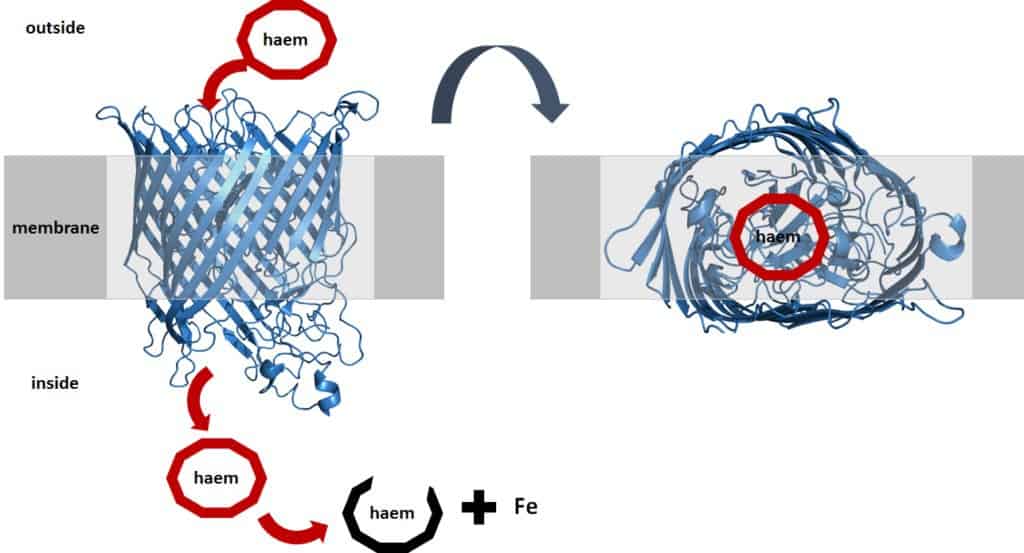
3 ways to steal our iron
A new study just found that my beloved bacterium of interest, Pseudomonas aeruginosa, actually contains three of these transporters. And all of these import iron into the cell. In this study, the researchers tried to understand why this bacterium would need three of these haem systems. Interestingly, they all seemed to have the same function – to find and import haem and thus iron.
The first thing they saw was that the bacterium produces any of these transporters only when it lives in a surrounding where there is little iron available. If a bacterium finds lots of iron, it takes it up easily without the need of these specialised transporters that cost a lot of energy.
Next, the researchers grew the bacteria with either no haem, a little haem or lots of it, while always keeping the iron low. Now they wanted to test under which conditions the bacteria would produce any of these three transporters.
When the cells grew with no or only a little haem, the bacteria produced one of the three systems, the so called Phu system. By producing this system even in the absence of haem, the bacteria prepare themselves for when haem comes along. Then they can immediately take it up and use it.
However, the Phu system did not work very efficiently when the bacteria grew with lots of haem. In this case, the bacteria produced the other two systems. These are the Hxu and the Has systems.
Why so many?
The Hxu and the Has systems are special transporter systems. And these are very similar to those that we discussed in the article “Bring in the iron”.
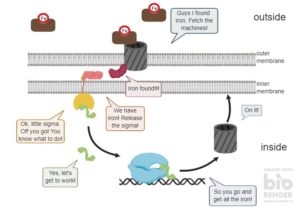
They contain the same barrel that you can see above. But the transporters are also involved in information sensing and messaging. This means that they not only take up the haem. These transporters also tell the bacterium that there is haem on the outside.
Like this, the bacterium knows that it is inside a host, as the human body. Because nowhere else would a bacterium find haem and especially in this amount.
Now that the bacterium knows that it is inside a host, it can produce all the heavy machinery to invade the host or make it sick.
Bacteria are always ready to find iron
But why does the bacterium need two of these haem transporting systems? Likely, as a safety net in case, one of them fails.
The researchers indeed found that the two systems can replace each other if one of them was missing. So with this, the bacterium makes sure that in any case the haem will be taken up and the iron used.
Now, the next step for the researchers will be to try to find drugs that can inhibit any of these transporters. This would cut off the messaging process and the bacterium would not know that it is currently inside a host. Then, it would not activate its heavy machinery to make us sick.
In all, this study told us a lot about how bacteria learn about their surrounding and showed us a new alternative to fighting these nasty bugs!
Take away from this week:
- Bacteria learn about the presence of haem in their surroundings to know they found a host
- Bacteria can use our haem to extract iron for their metabolic processes
- These transporters represent promising targets for anti-microbial drugs


4 Responses
VERY interesting
The barrel is upside down