What do you do when you are hungry?
You go to the fridge and get something to eat or you call your favourite pizza delivery service and order your favourite pizza.
Also, bacteria get hungry sometimes. But as you can imagine, they can’t really “see” what is in their fridge.
So, they need other ways to understand what they have in stock. And then they have to eat the food, which is also a bit more complicated when you don’t have a mouth.
Let’s look at one example of how bacteria eat: how they sense and eat iron.
Why do bacteria need iron?
All living organisms need iron to live, breathe and gain energy. So do bacteria.
However, iron is not just floating around everywhere, because free iron is actually highly toxic for cells. This is why all living organisms keep iron bound to specific iron vehicles.
You can imagine this as a pizza box. Inside the pizza box is the iron and the box itself protects the iron and keeps it from floating away and binding to other things.
Now, each organism has its very own unique pizza box for iron. For example, our bodies keep iron in their boxes so that bacteria cannot grow inside us. However, pathogenic bacteria found some sneaky ways to steal this iron.
Since iron is usually protected by these boxes, bacteria had to learn how to “see” iron even inside these boxes. This means bacteria had to learn to sense iron in their environment.
The tricky part for bacteria is that the information about the iron is on the outside. So, there are membranes that keep the iron and the information about it away from the bacterium. And this is where the pizza boxes come and help.
Bacteria steal pizza and sense iron
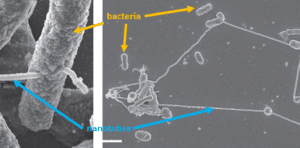
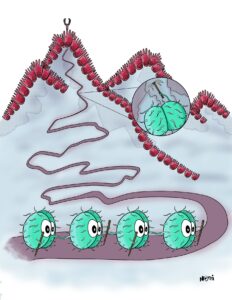
Also, bacteria have their own and unique pizza boxes that they use to sense and eat iron. To do so, bacteria send out empty pizza boxes. These are the vehicles in the picture below in brown with the red Fe.
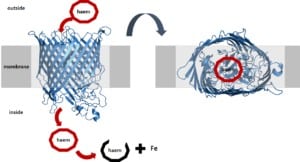
These pizza boxes can find iron far away and bring it back to the bacterium. A specific transporter (the grey tube) then transports the pizza box with the iron into the cell.
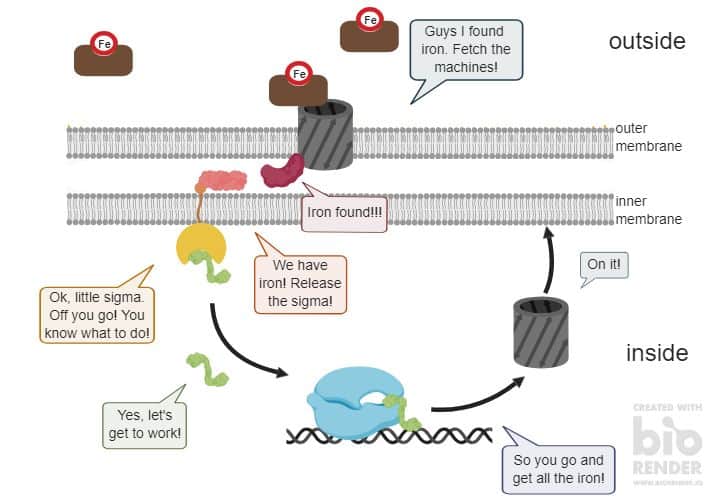
The bacterium now uses this transport process to tell the cell that it found iron on the outside. Otherwise, the cell would not know about this. Remember, bacteria have no eyes and don’t know that there is pizza on the outside.
The transporter is a tube that goes through the outer membrane. And on the inside, the transporter connects to another component, which looks like the little red bean.
This red bean is a messenger that hangs around between the two cell membranes. Here, it waits to receive the signal to go and find its counterpart – the pink bean – on the inner membrane.
This pink bean is bound to the yellow pacman that sits on the other side of the inner membrane. The yellow pacman is an inhibitor. It inhibits and keeps hold of a sigma factor, the green worm thingy.
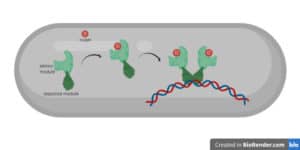
So, once the pink bean gets the signal from the red bean that there is iron on the outside, it cuts the yellow pacman. This means the pacman cannot bind and inhibit the sigma factor anymore so it is set free. Now, the sigma factor can go into the cell and bind its target – the big blue machine.
Sensing iron and making more transporters
The blue machine is the RNA polymerase, which is the cell factory. This one is responsible to initiate protein production from DNA. And the sigma factor is essential in this whole process.
It helps the RNA polymerase to find specific genes that are needed at a specific moment. In our example, the sigma factor shows the RNA polymerase the gene for the iron transporter.
Now, the cell knows it needs to produce more iron transporters and send them to the outer membrane. Here, the transporter waits for more iron-filled pizza boxes to transport into the cell. And with this circle, bacteria sense iron in their environment.
A complex game with one goal – find iron!
With this rather complicated mechanism, bacteria sense iron so they understand that iron is around. If there is no iron, the bacterium makes sure it only produces iron transporters when needed as they cost a lot of energy to produce. At the same time, the bacterium assures that it captures as much iron as possible when it is actually available.
So this is how bacteria make sure that they know exactly what is going on in their surrounding. While we call the pizza delivery service to bring us a pizza box filled with pizza, bacteria send out some empty pizza boxes and hope that some of them come back filled with delicious pizza.
It is probably not more efficient than our way, but what can you do when you don’t have a phone to directly order your pizza.



6 Responses
Bacteria with a personality in the picture…cool idea :)
Very intresting way inrtroducing that littel fun world keep up the great work sarah
Great work! Brain smart delivery. Narrative form much easier to assimilate. Illustrations memory builders as well. I commend you. Retired after 29 1/2 years in FL.
Thanks a lot for your comment and I am glad you find it helpful :)