All living organisms need energy.
Energy to grow, to move, to fight, to produce stuff and also to reproduce.
Generally, living organisms get this energy from food. It fuels us, just as it fuels animals, plants and bacteria.
But where exactly is this energy in food? How do bacteria and other living organisms access this energy? And what do they do if their favourite food is not around?
To answer these questions, let’s look at how molecules store energy.
How do living organisms gain energy?
Each chemical bond between atoms contains energy. Hence, a molecule that is made of many atoms and thus many chemical bonds, contains energy. When such a chemical bond opens, it releases energy in the form of electrons.
Depending on the kind of chemical bond within the molecule, these electrons can have higher or lower energy levels. Thus, they contain more or less energy.
So, to obtain energy from molecules, organisms need to break apart molecules and extract the electrons with high energy. But this is not as easy as it sounds. Chemical bonds are quite tight and it actually requires energy to break them open.
Hence, organisms need to have the right sets of proteins that can break open specific chemical bonds in molecules. These kinds of proteins are called enzymes. So, only if an organism has enzymes to break apart glucose, it can use glucose to extract its electrons and obtain energy.
Interestingly, most organisms do exactly that. They break apart glucose into smaller products and take the freed electrons. In that case, glucose is the so-called electron donor.
Now, these electrons need to go somewhere, since they are full of energy. So, organisms save this energy by transferring these electrons onto other molecules. These molecules have lower energy levels, hence they like to take up electrons. We call these molecules electron acceptors.
But finding the right electron acceptor is not as easy as it sounds.
The many steps from an electron donor to an electron acceptor
Imagine you stand on a high wall and want to get down onto the ground. You could take one big jump to reach the ground. But then you would risk that this high fall would give you so much energy that you might break your knees.
So, you could take a set of stairs, that brings you to the ground in multiple steps. Each step only releases a small chunk of energy but they would definitely not hurt you.
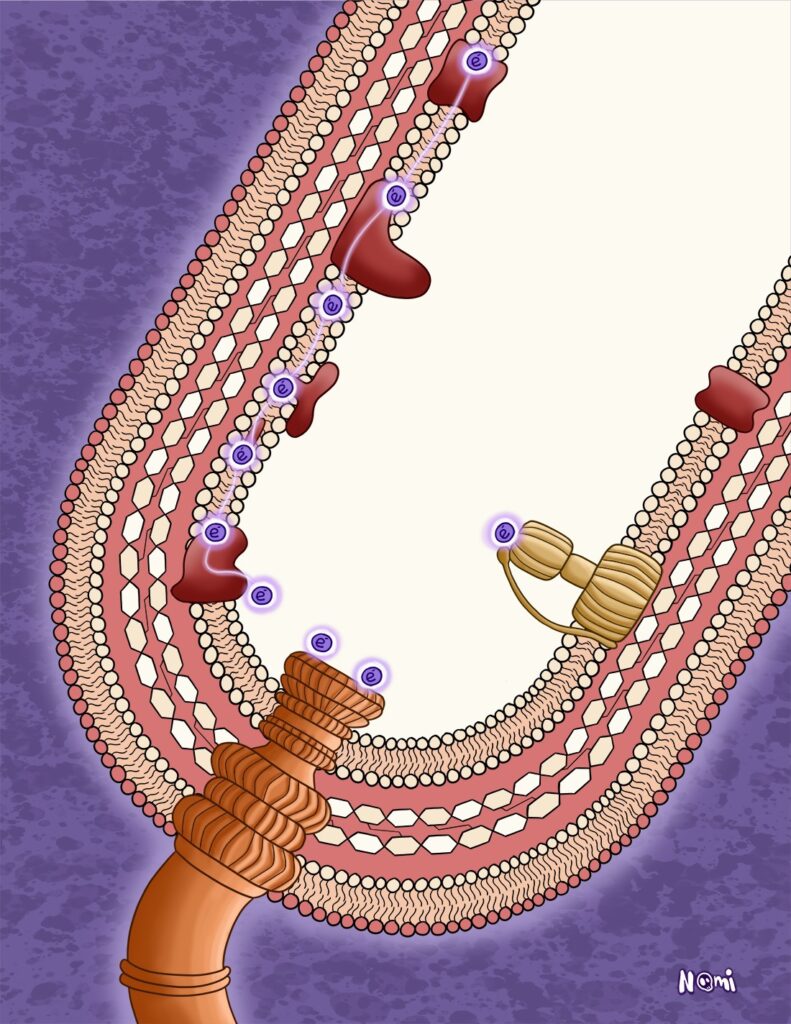
It is the same with electrons from donors with a lot of energy. Transferring these electrons to a final electron acceptor would free up too much energy at once. This could actually burn a cell. Hence, organisms transfer these electrons onto intermediate electron acceptors.
Each of these transfer steps only releases a small chunk of energy that keep organisms warm but also fuel cellular processes. In bacteria, these transfer processes happen in their membranes, where the released energy is directly used.
Here, the released electrons energise flagella so that bacteria can swim. Electrons can also activate transporters so that bacteria can import or export stuff. Not needed electrons and their energies are stored in energy-saving molecules like ATP.
This whole process of electron transfer from a donor to its final acceptor is generally what researchers call cellular or – more specifically – bacterial respiration.
Which molecules do bacteria use for cellular respiration?
Cellular respiration fuels most living organisms. And glucose is a molecule with one of the highest energy levels. Hence, breaking down glucose to extract its electrons is the most common in living organisms.
Animals do it. Fungi do it. So, bacteria are no exception to it.
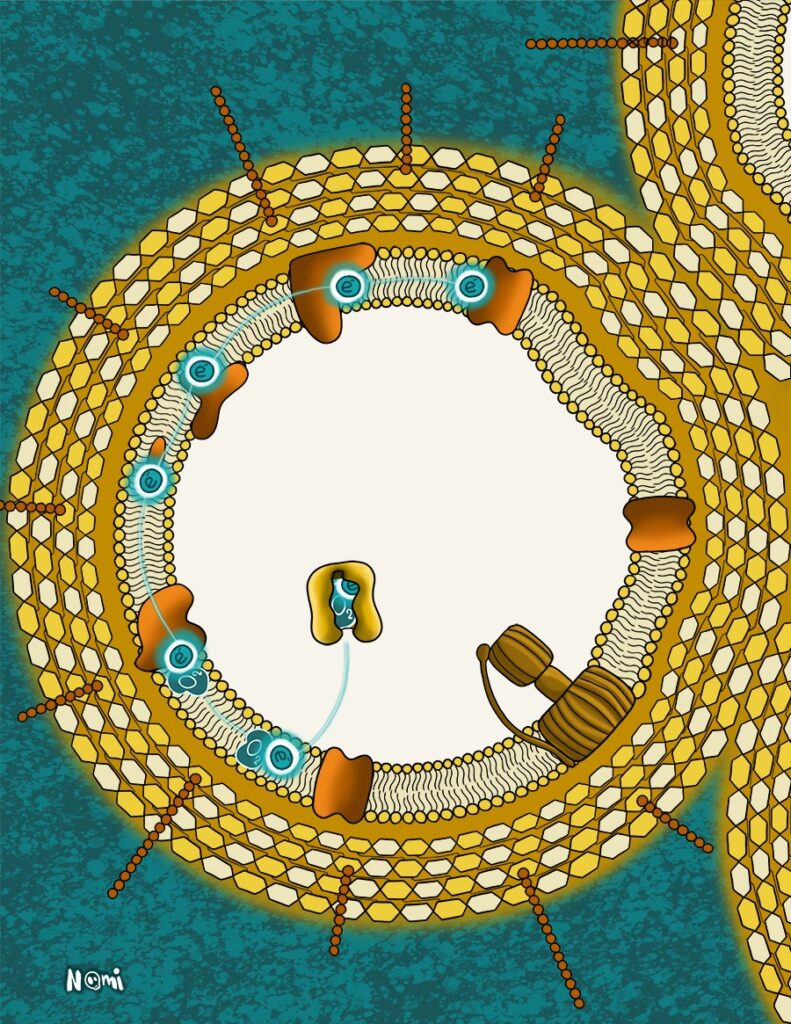
And as a final electron acceptor, most organisms use oxygen. This molecule has a very low energy level and is basically everywhere so most organisms transfer their electrons to it.
This is what we call aerobic respiration (which is what we generally do as well). But it comes with great risk.
The downside of aerobic bacterial respiration
As soon as an oxygen molecule is fuelled with just one electron, it becomes hyperreactive. Such a semi-activated oxygen molecule can basically react with any compound in a cell and damage it.
This is what makes aerobic respiration quite dangerous. So, every organism aims to hide these reactive oxygen molecules in the membrane.
Yet, it can happen that such a reactive oxygen molecule escapes the membrane. In this case, a special protein binds it and breaks it apart. So, every organism that does aerobic respiration has this same kind of protective protein to get rid of reactive oxygen molecules.
This means that whenever scientists find a new microbe, they first test whether this new microbe has these proteins. To test this, they add a bit of hydrogen peroxide to the bacterial colony. When bubbles come out of the bacteria, it means that they do aerobic respiration. In this case, they have the enzymes to break apart the reactive oxygen molecule and produce oxygen from it.
Which other molecules can bacteria use as energy source?
As we said, most bacteria use glucose as an energy source for cellular respiration. However, there are also many fancy exceptions. And these exceptions make the bacterial – and microbial – world so colourful and diverse.
While many bacteria can extract electrons from many different organic acids and amino acids, some use sulphur compounds. Some bacteria also break apart greenhouse gases like methane, carbon monoxide or even hydrogen gas. Since these bacteria might be helpful in tackling our climate problems, they are of particular interest to researchers!
What does bacterial respiration look like without oxygen?
We surely need our oxygen for respiration. Yet, many bacteria and fungi can live with only small amounts of it or even no oxygen at all.
In this case, they do anaerobic respiration. This basically means that they don’t transfer their electrons to oxygen as an electron acceptor.
Instead, many bacteria have enzymes to transfer their electrons to different electron acceptors. And these depend on what the bacteria have available around them. These electron acceptors can be nitrate or sulphate compounds, salts like arsenate or even metals like iron and gold.
Also, in many microbes, anaerobic respiration is closely related to microbial fermentation. In this case, the bacteria break apart glucose but produce molecules that do not require oxygen.
Just think about yeast that produces ethanol in beer and wine. Or lactic acid bacteria in your sauerkraut and yoghurt that produce lactic acid to make the food more acidic. Lastly, there are fungi like Zymomonas mobilis that produce huge amounts of ethanol from glucose.

Bacterial respiration makes the microbial world diverse
As we have seen, bacteria learned to use various sources to gain energy. They created the right enzymes to extract electrons from fancy high-energy molecules.
And then they learned to transfer these electrons onto even fancier molecules to gain the most energy. Some of these processes even involve bacteria producing shiny gold!
In my opinion, these truly amazing superpowers make the bacterial world so incredibly colourful and fascinating!